Biopharmaceuticals: New Hope for Patients
A new age has dawned in the pharmaceutical sector. A large number of biological medical products are being developed to market readiness. They have the potential to treat nearly every illness, and offer hope to patients who have conditions previously considered non-treatable or ones that are very rare. For these innovations to benefit everyone, their development processes need to be improved and their remuneration models reassessed. That is the conclusion of our author, Dr. Roman Hipp, a Senior Partner at Porsche Consulting.
07/2024
Metachromatic leukodystrophy (MLD) is a rare, genetic metabolic disorder whose diagnosis used to be tantamount to a death sentence. A mutated gene leads to the progressive destruction of the insulating layer around nerve fibers in the brain, which means they can no longer transmit signals. Affected individuals gradually lose motor skills such as the ability to move, talk, speak, and swallow. In the absence of treatment, life expectancy is low.
Medications “know” patients
However, a treatment — or more precisely, a gene therapy — for this disease has recently been developed. A patient’s stem cells are removed and altered in a lab to give them a functional, intact copy of the defective gene. The stem cells are then infused back into the body, where they can reproduce in bone marrow. The results are astounding. So too are the costs, currently with a price tag of 3.91 million euros for a single treatment, as reported by Orchard Pharmaceuticals.
This example highlights the innovation that biopharmaceuticals can bring, as well as the cost challenges. Traditional pharmaceuticals are often effective for large numbers of people and, if their patents have expired and generics come to market, often cost very little to relieve a headache or suppress a cough, for instance. Biopharmaceuticals, on the other hand, are noted for their targeted ability to treat or eliminate what are often rare conditions. Examples of these developments include bispecific antibodies for cancer immunotherapy, using the body’s own immune cells to combat tumors, and cellular scissors to repair defective genes such as the above-mentioned cause of MLD. In each case, the strength lies in its more personalized approach. Each patient receives treatment that is tailored precisely to his or her condition. Estimates by Porsche Consulting suggest that the market for biopharmaceuticals will nearly double by 2030.
From big pharma to start-ups, everyone wants a piece of the pie. But there are enormous hurdles on the road to success in the biopharmaceutical business. Manufacturing processes are more complex than those for traditional chemical agents. And the new treatments are already facing a series of setbacks in their development.
A real urgency
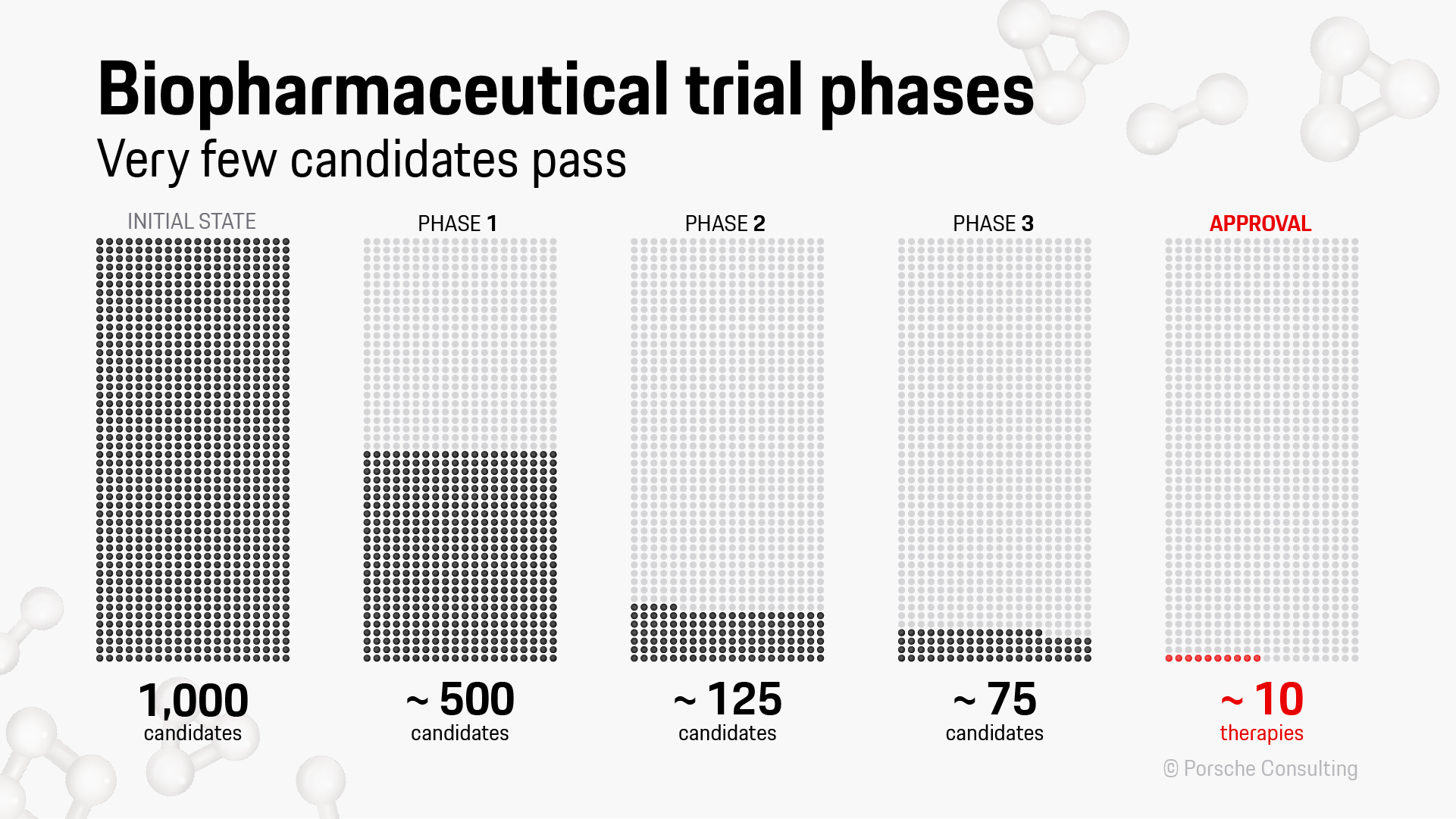
An analysis by Porsche Consulting clearly shows that of 1,000 molecules considered promising candidates for new medications, only around half of them survive the first phase of clinical studies. All the others are rejected, usually because of major side effects. In the second phase, which tests the dosage and efficiency of active substances, the cohort loses around another three-quarters of its members. And in the third phase, with trials on thousands of people, another 40 percent of the candidates do not pass muster. Ultimately only around ten of the initial 1,000 agents will make it through the approval process, ready for use as therapies. This often takes a decade or more, and can devour billions of euros.
These figures are not specific to biopharmaceuticals, but also reflect development challenges faced by drug companies that make medications based on chemical molecules. But they indicate the magnitude of development costs in the sector. How can the processes become cheaper and faster? One possibility is to make greater use of artificial intelligence (AI). An analysis from the journal Drug Discovery Today has shown that 80 to 90 percent of candidates selected by AI make it through the first phase of clinical studies. That is an enormous increase over the number selected “manually” by research personnel. Targeted investments in AI therefore make sense for pharmaceutical companies. Another possibility lies in the phase before human trials and consists of replacing the obligatory, albeit error-prone, tests on animals with organ-on-a-chip experiments. For this, human cells are cultivated in a lab to make small amounts of tissue suitable for drug tests — an approach that will depend on favorable regulatory frameworks in the future.
It is also important to entrust the development of new medications to diverse teams. Not only R&D specialists should be involved right from the start but also representatives from manufacturing, marketing, regulatory affairs, and quality management. The combined perspective helps to save time and costs because it ensures that only the most promising candidates are admitted to clinical studies in the first place.
Looking at this from a global perspective, there is potential for speeding up the pace of these processes. International companies often reach phase 3 or beyond in advanced biologics, while German players are still in phase 1 or 2.
That being said, German companies can learn from other companies’ best practices — particularly of U.S.-based enterprises — and quickly catch up. For this, they need to place a higher priority on innovative technologies in their R&D departments, ideally in market sectors where they are already competitive. Strategic outsourcing of individual activities can further accelerate progress.

Paid for success
Every larger company also has to explore whether it wishes to purchase external innovations and integrate them into its existing organizational model, for example in the form of collaborative projects with spin-offs from universities or research institutions, or whether it should build up the requisite expertise internally. The experts at Porsche Consulting can provide valuable assistance in selecting the right targets,and establishing new value pools.
Innovations are also needed in financing. If a treatment costs millions of euros, it will not be available to everyone — it is simply too expensive. And the solution? We need to reassess remuneration models and break free of tradition. Currently, the sales volume of a drug is what is rewarded. Another possibility would be to compensate purely on the basis of outcome — and pay producers not for every drug sold but for every treatment that succeeds.
Without a doubt, patients will benefit from biopharmaceutical innovations. And the faster these medications can go onto the market and the more favorable their prices, the more human lives they will be able to save.
